I recently posted on LinkedIn about a favourite subject of mine, deliquescence. You may think this is a bit weird to have enjoyment in such thing, but it simply fascinates me from all sorts of angles.

Deliquescence – What Is It?
To begin with, deliquescence is a perfectly natural process in some materials that adsorb moisture. There is a natural tendency for materials to adsorb and release moisture depending on the surrounding relative humidity (%RH). Some materials that have a high solubility in water adsorb moisture from the air and the molecules at the surface dissolve in this moisture. As more moisture is adsorbed, more of the material dissolves and this can continue until all the material turns into a liquid solution of the substance.
In Nature this phenomenon is exploited by some organisms for their survival. House mites will only thrive above a certain level of humidity and that is determined by deliquescence of a Na and K salt combination and the liquid draining into the mouth of the mite. The Atacama Desert, located in northern Chile, is accepted as being the driest and oldest part of our planet. Microbiologists have studied the bacteria and fungi from this region since the 1960’s. One area of scientific research has been studies on microorganisms in halite crustal deposits in evaporated lakes and salts within rocks where moisture can penetrate. Deliquescence of these salts is transient occurring when moist air from the Pacific Ocean is carried in land, but sufficient to provide a source of water for microorganisms.
Rock Salt – An Unexpected Discovery
So how can we use this phenomenon of deliquescence in practical ways? Let us take a look at what happens to rock salt. In this example a candle holder made from rock salt, but this is also applies to rock salt lamps. Compositional analysis of the well-known Pink Himalaya Rock Salt shows it to be about 38.6% sodium. A quick chemical calculation tells us that NaCl is made up of 39.3% sodium. The difference in rock salt from pure NaCl is less than 1% of other minerals.

A Christmas present of two rock candle holders were left sitting on a glass table. They both ended up surrounded by a clear liquid. It took several sheets of kitchen roll to mop up these little pools. Magically this liquid seemed to have appeared out of thin air!
The truth is that it did come out the air, but not by magic. A saturated solution of NaCl is accepted by IUPAC as a standard for 75 %RH with which to calibrate humidity instruments. However, 75 %RH is also the humidity at which solid NaCl begins to adsorb moisture from the air.
Application Of Knowledge
The outside humidity was quite high, so a practical experiment was performed. I left the salt candle holder outside on a plastic chair for a few of days when there was no rain forecast. In the photo you can see the liquid produced from running off the candle holder. At the edges and within the liquid are white spots from the salt recrystallizing as water then evaporates. The same effect happens at salt lakes and hot salt springs that gives the salt deposits that the candle holder was probably made from in the first place. For this to occur with the salt candle holder the outside humidity would have to be above 75%.

The Relative Humidity of Deliquescence
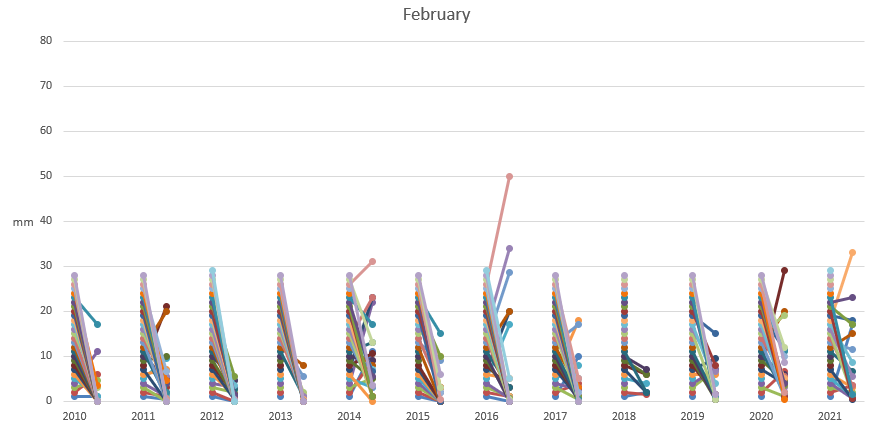
Observing the deliquescence in the lead photo, would you not like to know at what humidity this was going to occur? One technique for obtaining this information is Dynamic Vapour Sorption (DVS). This technique is generally regarded as the ‘gold standard’ for moisture studies. Performed at constant temperature, a moisture sorption isotherm of your API undergoing deliquescence may appear as a material with little or no weight gain and then show an inflection at a particular relative humidity.
I was kindly granted permission to use the picture below of DVS graphs, known as moisture isotherms. Weight gain and loss in a material as the %RH increases and decreases are shown for a substance that undergoes deliquescence. There may be other interpretations, but my view is that between 70-80 %RH an inflection in the curve is caused by the substance starting to deliquesce as it adsorbs moisture. In the drying cycle of reducing %RH the substance returns to a solid state at 70-80 %RH. Note that the substance having been exposed to moisture prevents its released at lower %RH and the same starting %RH value is not achieved. One reason could be that a new crystalline state trapping in water molecules has been formed and this substance is displaying polymorphism.
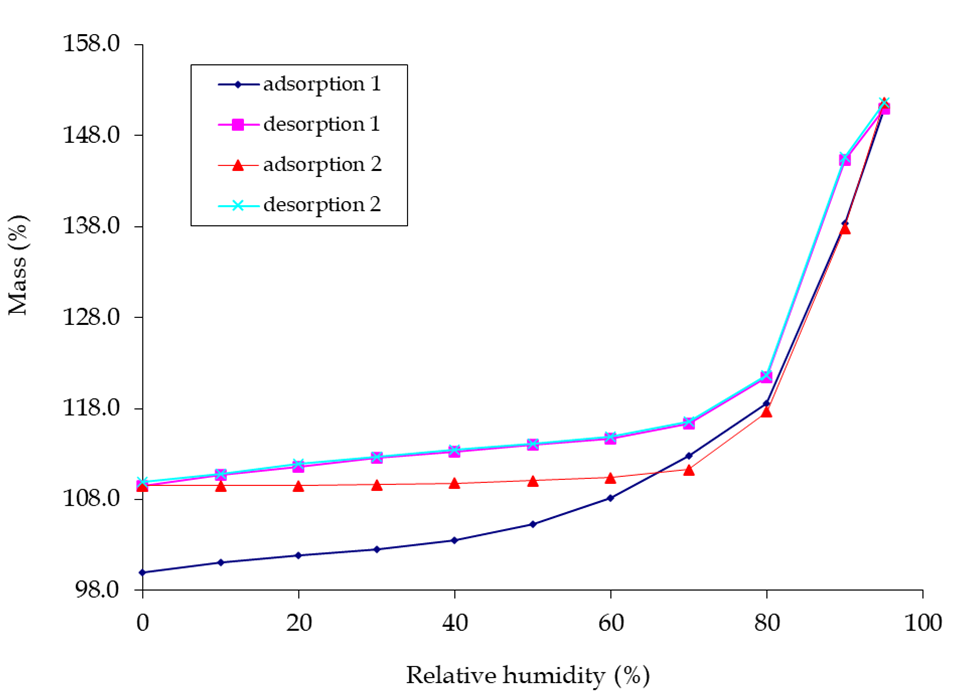
Moisture Profiling
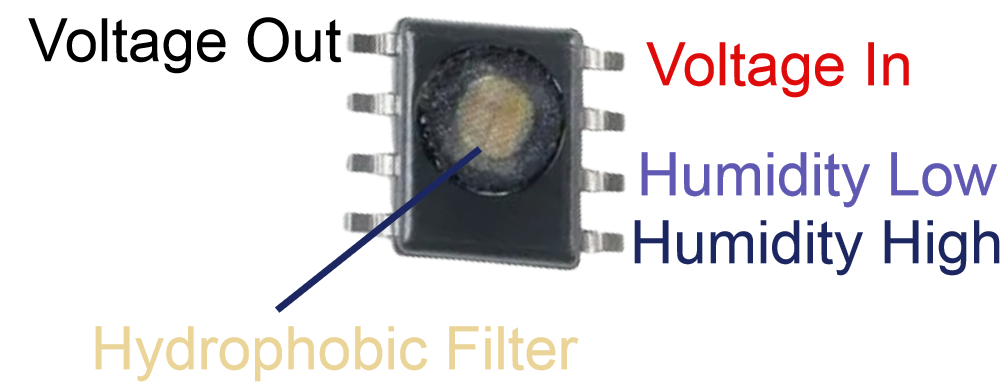
Relequa’s MP-1000 Moisture Profiling™ System can performed the task of identifying the deliquescence relative humidity at ambient temperature. The API in the lead photo was found upon testing by Moisture Profiling™ to have a Water Vapour Equilibrium Point (WVEP) of 29%. To avoid moisture uptake and prevent deliquescence in this case, the API should be handled in an atmosphere below 29 %RH. If we had tested the rock salt of the candle holder, we would find that the WVEP would be around 75%.

In less than an hour, the WVEP of an API can be determined using the MP-1000 Moisture Profiling™ System.